|
Symbol |
Title |
Description |
Standard Title/ Reference Title |
Reference |

|
Manufacturer |
Indicates the medical device manufacturer |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.1 |

|
Authorised representative in the European Community |
Indicates the authorized representative in the European Community/ European Union |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.2 |

|
Date of manufacture |
To identify the country of manufacture of products |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.3 |
|
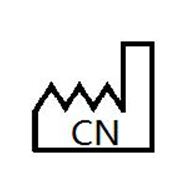
Country of manufacture
|
To identify the country of manufacture of products |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.11 |

|
Use-by date
|
Indicates the date after which the medical device is not to be used |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.4 |

|
Medical device
|
Indicates the item is a medical device |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.7.7 |

|
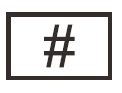
Unique device identifier
|
Indicates a carrier that contains unique device Identifier information |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.7.10 |
|
Model number
|
Indicates the model number or type number of a product |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.10 |

|
Batch code
|
Indicates the manufacturer’s batch code so that the batch or lot can be identified |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.5 |

|
Catalogue number
|
Indicates the manufacturer’s catalogue number so that the medical device can be identified |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.6 |

|
Serial number
|
Indicates the manufacturer’s serial number so that a specific medical device can be identified |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.7 |

|
Importer
|
Indicates the entity importing the medical device into the locale |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.8 |
|
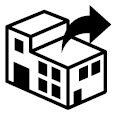
Distributor
|
Indicates the entity distributing the medical device into the locale |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.9 |

|
Sterile
|
Indicates a medical device that has been subjected to a sterilization process |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.1 |

|
Sterilized using ethylene oxide
|
Indicates a medical device that has been sterilized using ethylene oxide |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.3 |

|
Sterilized using irradiation
|
Indicates a medical device that has been sterilized using irradiation |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.4 |

|
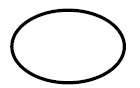
Sterilized using Steam or Dry heat
|
Indicates a medical device that has been sterilized using steam or dry heat |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.5 |
|
Single sterile barrier system
|
Indicates a single sterile barrier system |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.11 |
|
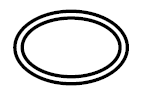
Double sterile barrier system
|
Indicates two sterile barrier systems |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.12 |
|
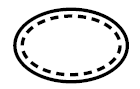
Single sterile barrier system with protective packaging inside
|
Indicates a single sterile barrier system with protective packaging inside |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.13 |
|
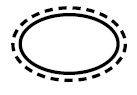
Single sterile barrier system with protective packaging outside
|
Indicates a single sterile barrier system with protective packaging outside |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.14 |

|
Do not resterilize
|
Indicates a medical device that is not to be resterilized |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.6 |

|
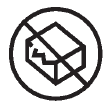
Non-sterile
|
Indicates a medical device that has not been subjected to a sterilization process |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.1.7 |
|
Do not use if package is damaged and consult instructions for use
|
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information NOTE 1 This symbol can also mean “Do not use if the product sterile barrier system or its packaging is compromised”. NOTE 2 For products that do not have instructions for use, the recommendation to consult them does not apply. |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.2.8 |
|
Keep away from sunlight
|
Indicates a medical device that needs protection from light sources |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.2 |

|
Protect from heat and radioactive sources
|
Indicates a medical device that needs protection from heat and radioactive sources |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.3 |

|
Keep dry
|
Indicates a medical device that needs to be protected from moisture |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.4 |
|
Lower limit of temperature
|
Indicates the lower limit of temperature to which the medical device can be safely exposed |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.5 |
|
Upper limit of temperature
|
Indicates the temperature limits to which the medical device can be safely exposed |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.6 |
|
Temperature limit
|
Indicates the temperature limits to which the medical device can be safely exposed |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.7 |
|
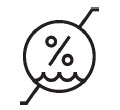
Humidity limitation
|
Indicates the range of humidity to which the medical device can be safely exposed |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.8 |
|
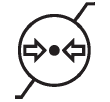
Atmospheric pressure limitation
|
Indicates the range of atmospheric pressure to which the medical device can be safely exposed |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.3.9 |

|
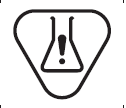
Biological risks
|
Indicates that there are potential biological risks associated with the medical device |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.1 |

|
Do not re-use
|
Indicates a medical device that is intended for one single use only |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.2 |
|
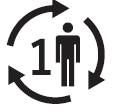
Single patient multiple use
|
Indicates a medical device that may be used multiple times (multiple procedures) on a single patient |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.12 |

|
Consult instructions for use or consult electronic instructions for use
|
Indicates the need for the user to consult the instructions for use |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.3 |

|
Caution
|
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.4 |

|
Contains or presence of natural rubber latex
|
Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of a medical device |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.5 |
|
Contains hazardous substance
|
Indicates a medical device that contains or incorporates human blood or plasma derivatives |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. |
ISO 15223-1, Reference 5.4.10 |

|
Prescription use only
|
Labeling; Prescription devices |
21 CFR 801.109 |
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |

|
CE-Mark
|
Indicates manufacturer declaration that the product complies with the essential/ general safety & performance requirements of the relevant European medical device, health, safety and environmental protection legislations. |
European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC). European Medical Device Regulation 2017/745 |
European conformity (CE) mark for Class I non-sterile medical devices. |

|
CE-Mark with notified body number
|
Indicates manufacturer declaration that the product complies with the essential/ general safety & performance requirements of the relevant European medical device, health, safety and environmental protection legislations. |
European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC). European Medical Device Regulation 2017/745 |
European conformity (CE) mark with Notified Body identification number for Class Im, Ir, Is, IIa, IIb, III medical devices. |